About the Synthesis of Water in a Water Bottle Movie |
This one is not for the faint of heart. I do not recommend it unless you have a lot of experience with this reaction and you now how to carry out a remote ignition with some kind of detonation cord. For the small quantity of reactants involved, this reaction is amazingly loud and destructive. I have compressed cylinders of industrial grade hydrogen and oxygen so the mixture is of high purity. I now fill the water bottle "over water" so I can see that I am getting close to a 2:1 ratio of the reactants. It is a very neat "teaching moment" to have the students come up with the balanced equation and generate the ratio required for the best reaction. You can read more about my set-up and see some still frames of a past experiments from this link. Below is the set-up for the 2001 experiment that is in the movie.
The ring stand was set up with two ring clamps.
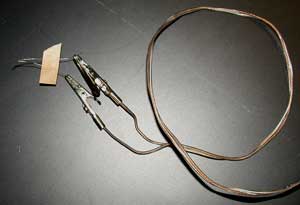
This is my detonation cord - ordinary stereo wire with alligator clips added.
A rocket igniter is attached to the wire.
The top clamp was to hold the bottle in place and the bottom clamp supports the wire so that the igniter rests in the mouth of the bottle.
The force of the explosion broke the top ring into 4 pieces. Even more amazing was that the explosion snapped the lower clamp that was not in direct contact with the bottle.
The rigid plastic was no match for the speeding molecules. Keep in mind that this reaction was conducted outdoors and the detonation cord allows me to initiate the reaction from a distance. Hopefully these pictures demonstrate that one should respect chemical reactions even when relatively small amounts of ingredients are used. Go to the NASA web site and find out how much liquid oxygen and hydrogen are used in their launch procedure for the Space Shuttle. You can order a supply of plastic bottles for your own experiment online. You could try different types of plastic bottles and get different results from the experiment.
|